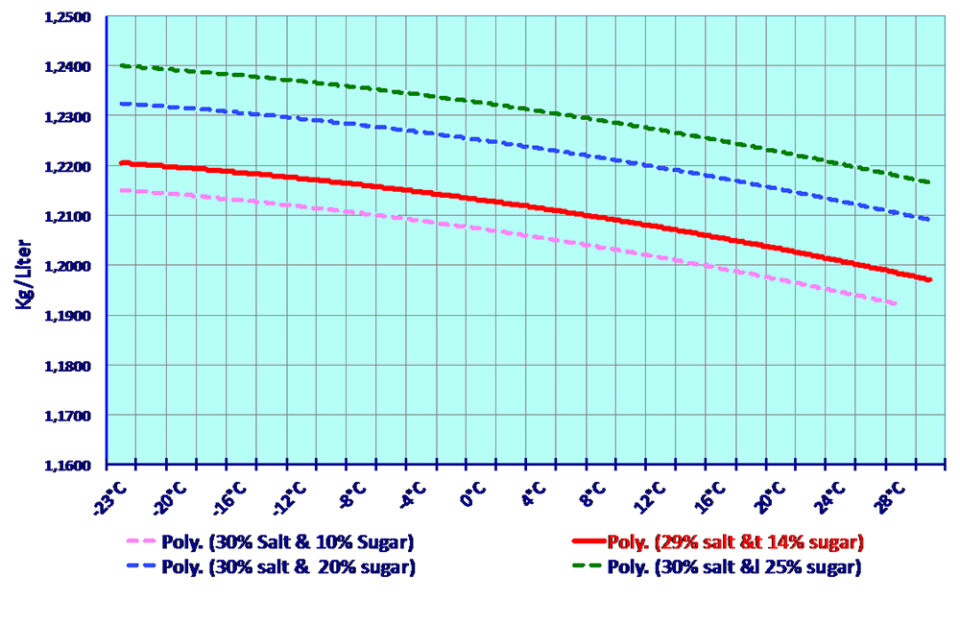
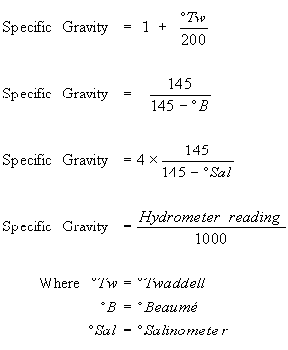The table below gives the density kg l and the corresponding concentration weight of sodium chloride nacl in water at different temperatures in degrees centigrade c.
Density of brine at room temperature.
Pressure volume temperature composition p v t x data for brines are required to establish optimum operating temperatures pressures and flow rates for the production of geothermal brine fields to minimize scaling and corrosion and to design intelligently turbines for production of electricity.
Correlations for lubricating oil density and temperature are calculated by use of tools based on astm d 1250 04 and ip 200 04 api manual of petroleum measurement standards chapter 11 physical properties data section 1 temperature and pressure volume correction factors for generalised crude oils refined products and lubricating oils.
Density of inorganic chlorides in water is plotted as function of wt mol kg water and mol l solution.
Saturated sodium chloride brine density solubility at various temperatures f c specific gravity sodium chloride brine wt lbs gal lbs gal 32 0 1 2093 26 34 2 652 10 07 50 10 1 2044 26 35 2 644 10 03 59 15 1 2040 26 40 2 647 10 03 68 20 1 1999 26 43 2 643 10.
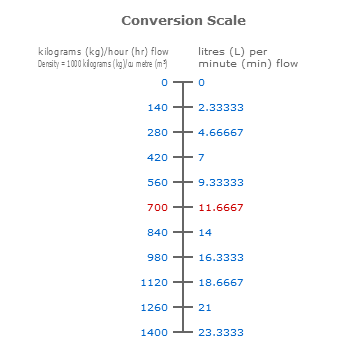
Input a temperature and density within the range of the table to calculate for concentration or input concentration to calculate for density.
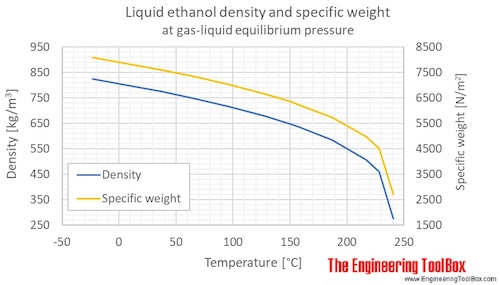
Density is the ratio of the mass to the volume of a substance.
Specific weight is the ratio of the weight to the volume of a substance.
Each colored line represents a lubricate with a given.
ρ m v 1 where ρ density units typically g cm 3 or lb ft 3 m mass units typically g or lb v volume units typically cm 3 or ft 3.
Brine is a high concentration solution of salt nacl in water h 2 o.
One of the prerequisites for.
In different contexts brine may refer to salt solutions ranging from about 3 5 a typical concentration of seawater on the lower end of solutions used for brining foods up to about 26 a typical saturated solution depending on temperature lower levels of concentration are called by different names.
All hydrometers are calibrated to be accurate at a given temperature.
Density of aqueous solutions of inorganic chlorides changes in density of aqueous solutions with changes in concentration at 20 c.
Density converter online density converter with commonly used units.
Linear regression by the method of least squares is used to curve fit saturated liquid enthalpy and density.
Because the density of fluids changes as their temperature changes if you don t measure your specific gravity at your hydrometers calibration temperature you re going to get an inaccurate reading.