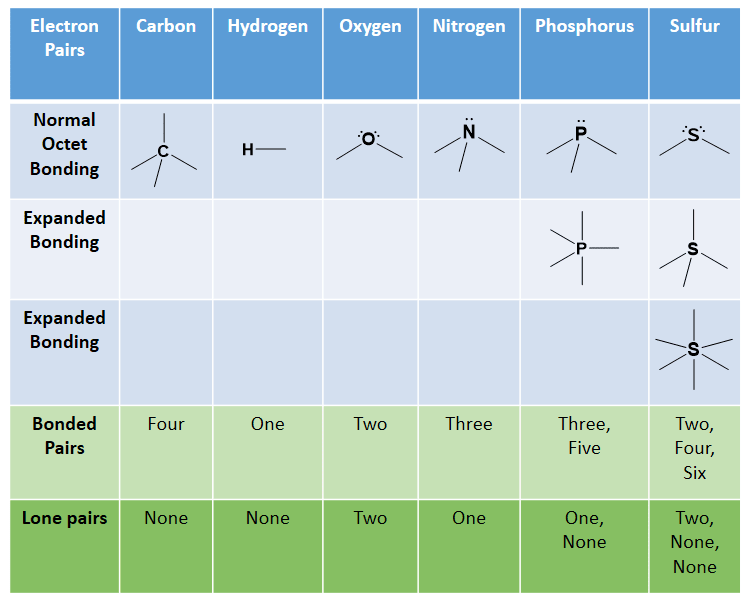
If possible explain with reference to their molecular structure shape and find homework help for.
Explain why water is a liquid at room temperature while methane is a gas.
Explain why water is a liquid at room temperature while methane is a gas at room temperature.
Well ionic substances are solid at room temperature they are generally soluble in water they have high melting and boiling points while covalent substances are generally liquid and gases at room.
Room temperature is defined as anywhere from zero to 100 degrees celsius.
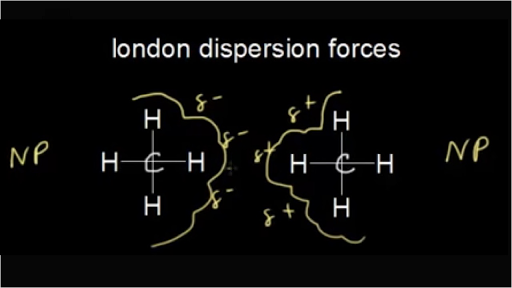
Because of the hydrogen bonding between water molecules water is a liquid at room temperature.
In other temperatures water can also be a gas or a solid.
An overall sticky effect is created.
Water changes easily between its three forms.
In chemistry we are learning about polar bonding and hydrogen bonding so i know the answer is related to this but i don t understand how.
Determine the formulas for each and figure out the types of intermolecular forces that exist.
Why is methane ch4 a gas and methanol ch3oh a liquid.
Water becomes a gas when the hydrogen bonds that form molecules move quickly.
Water becomes a solid or frozen when the molecules are slowed down.
Molecules like oxygen gas and nitrogen gas are gases at room temperature.
At room temperature methane ch4 is a gas but water is a liquid.
Get an answer for explain.